5 mins ago
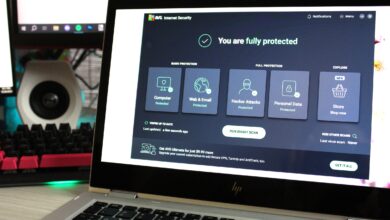
This killer AVG Closing antivirus deal is correct $18 for 2 years
5 mins ago
How we test USB-C cables at PCWorld
5 mins ago
uninstall McAfee antivirus
5 mins ago
Controversial Home windows 11 Commence menu ads delivery up rolling out
5 mins ago
Handiest password managers 2024: Offer protection to your online accounts
5 mins ago
With Firefly Image 3, Adobe objectives to integrate extra AI tools for diversified apps
5 mins ago
How vivid and proper files devices force effective first-procure together files activation
5 mins ago
As TikTok ban threatens steadiness in social media ecosystem, some manufacturers resolve into the fediverse
5 mins ago
Future of TV Briefing: Sneak look at ‘The Future of TV’ video sequence
5 mins ago